Clinical research refers to all scientific studies carried out on human beings to better understand diseases and to develop new treatments and diagnostic methods. This research is essential for better patient care in the long term
Before new treatments can be offered to all patients concerned, it must be ensured that they are effective and well tolerated. The development of a drug is a long and complex process. The new drug is first developed in a laboratory and then tested on animals (this is pre-clinical research). If the results of these tests are favourable, patients (cancer patients for the trials promoted by Unicancer) may then be offered to participate, with their agreement, in its evaluation. This evaluation, carried out with the collaboration of patients, is called a clinical trial.
12,248 patients enrolled in Unicancer-sponsored clinical trials in 2023.

Patient participation in clinical trials is an essential contribution to the discovery of new treatments and strategies that can benefit many people affected by the disease
Jean-Yves Blay – President d’Unicancer
The course of a clinical trial in 4 phases
To ensure patient safety and scientific rigour, clinical trials include several phases (4 in total), each designed to gather specific information about the new treatment.
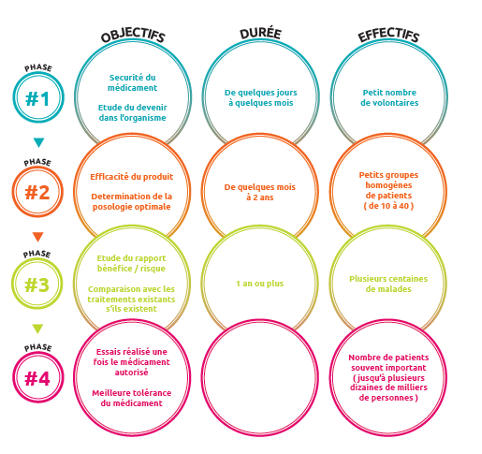
- Phase I trials are designed to assess the safety of a new drug in order to determine the recommended dose and in some cases to give first indications of efficacy. The treatment being evaluated is administered to a small number of patients (10 to 40).
- Phase II trials evaluate the effectiveness of a treatment. They usually require the inclusion of a larger number of patients (40-80 patients) to exclude the risk of drawing wrong conclusions about the efficacy of the treatment. This phase also allows to confirm and consolidate knowledge on the safety of the treatment
- Phase III trials are comparative trials. They allow the new treatment to be compared with the treatment normally used, known as the “standard treatment”. Two groups of patients are drawn by lot (randomisation), which makes it possible to create homogeneous and comparable groups (age, sex, characteristics of the disease, etc.): one group will receive the reference treatment, the other the new treatment. It is therefore not the doctor who decides which treatment to give to his patient. These trials require the inclusion of a large number of patients (several hundred) to establish a real difference between the treatments. If the data and results of these trials are in favour of the new treatment, this makes it possible to compile a registration file that will be submitted to the health authorities so that they can issue a marketing authorisation (MA), which authorises the marketing of the new treatment.
- When the drug is marketed, it is still subject to close monitoring called pharmacovigilance (Phase IV). Thus, any unexpected abnormal sign due to its administration is reported to the French National Agency for the Safety of Health Products (ANSM).
The patient does not participate in all phases in succession but is offered to participate in a trial in one of the four phases, according to very precise criteria.
Clinical trials objectives
Most often clinical trials are designed to evaluate a drug, however clinical trials can evaluate
- New drugs or drug combinations (to treat a disease, reduce side effects, improve quality of life, etc.)
- New ways of administering treatments (tablets instead of injections, etc.)
- New treatment techniques (new type of surgery or radiotherapy, etc.)
- New diagnostic techniques (new biological tests, etc.)
A well established regulatory framework, guaranteeing patient safety
The studies are submitted to 2 control institutions:
The Comité de Protection des Personnes (CPP) is an independent committee composed of people qualified in biomedical research and ethics: health professionals (doctors, pharmacists, nurses), epidemiologists, psychologists, lawyers, representatives of patient and health system user associations.
The CPP assesses whether the trial is scientifically and ethically acceptable, in particular the protection of the patients involved in the trial. The CPP looks in particular at the physical and psychological safety of patients by assessing the benefit/risk ratio, but also at the information received by patients before agreeing to take part in the trial and at the measures put in place to protect the patients’ personal data.
The Agence Nationale de Sécurité du Médicament (ANSM) assesses the safety, quality and proper use of the health products used during the trial, thus guaranteeing the physical safety of the patients involved in the trial.
Both committees must agree to the conduct of a clinical trial. If one of them considers that the conditions assessed are not acceptable, they are able to object to the trial being conducted.
How to join a clinical trial?
Patients participating in clinical trials thus have access to innovative treatments that are not yet available to all patients suffering from the same disease.
To participate in a clinical trial, patients must meet a number of criteria, called “eligibility criteria”. Each clinical trial is designed to answer a specific question, so the “eligibility criteria” are specific to each trial. Therefore, some patients will be offered to participate in a trial because of certain characteristics, such as the type or size of their tumour, while others will not be able to participate but will be eligible to enter another trial more appropriate to the characteristics of their disease.
Intensified patient monitoring during clinical trials
Patients who enter a clinical trial benefit from a specific follow-up predefined by the terms of the trial. Indeed, a clinical trial provides for monitoring of the patient throughout the course of the trial, in order to verify the effectiveness and tolerance of the treatment. For this purpose, the patient benefits from specialised care with more frequent follow-up visits and additional analyses compared to the clinical care usually carried out for the follow-up of patients outside clinical trials.


