- RWD

Urogenital Tumor Study Group (GETUG)
Contact us
GETUG develops cancer research programs in the various locations of the urological and male genital systems.
Description
Established in 1994, GETUG has become the national referent cooperative group in its field.
GETUG brings together experts from several specialties, such as medical oncologists, radiotherapists, urologists anatomo-pathologists and statisticians, working on urogenital cancers. This cooperative group of French experts defines the research areas and develops the corresponding clinical trials.
In 2010, a partnership was established with AFU (Association Française d’Urologie) which aims at jointly conducting national and international phase II and III therapeutic trials
It reinforces a collaboration that was initiated in 2004, between GETUG and AFU, which started with the GETUG-AFU 15 study comparing the combination of hormonal therapy with docetaxel to hormonal therapy alone in hormone-sensitive metastatic prostate cancer.
The French National Cancer Institute (INCa) accredited the intergroup GETUG-AFU called ICF-URO in 2015 with the integration of CeREPP (Centre de Recherche sur les Pathologies Prostatiques et urologiques) and renewed the accreditation of the intergroup GETUG-AFU-Alliance in 2018.
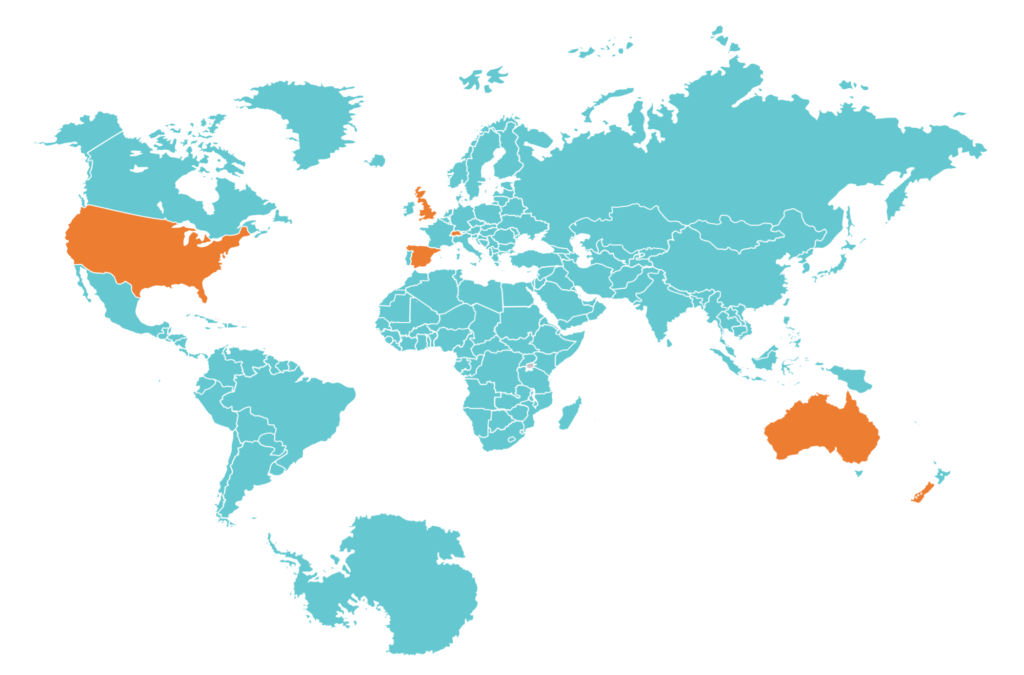
GETUG collaborates with has a very large network of university hospitals, general hospitals, CLCCs and private institutions in France and abroad and develops research projects of international dimension by establishing links with institutions in Europe and the United States (SOGUG, ICORG, EORTC, SAKK, MD Anderson, RTOG, UCL …etc).
Since its creation, GETUG has been conducting 35 national and international multicenter clinical trials sponsored by Unicancer, 5 international clinical trials in collaboration with European partners (SAKK- EORTC and UCL). In addition, more than 30 clinical trials sponsored by other French structures have been labelled by GETUG.
Communications and publications

10 publications en 2021

14 communications at international congresses (7 oral communication)
The communications of the results of the prospective studies conducted by GETUG at major international congresses and in “high impact factor” journals have enabled the group to acquire an strong international visibility over the years and to conduct European-scale trials (PEACE program – Prostate cancer Consortium in Europe) , such as the PEACE 1 and PEACE 2 directed by Professor Karim Fizazi.
GETUG collaborates also with many pharmaceutical companies such as ASTELLAS – BAYER – BMS -FERRING – IPSEN – JANSSEN- ROCHE – SANOFI that provide experimental treatment as well as grants to perform the clinical trials.
GETUG IS A KEY INTERNATIONAL ACTOR IN CLINICAL RESEARCH ON UROGENITAL CANCER.

41 national and international multicentre clinical trials funded by Unicancer since the creation of GETUG
11 studies under recruitment, 6 of which are international

405 patients included in 2021 in trials sponsored by Unicancer
Synopsis of the studies under recruitment
- GETUG-AFU 27 TIGER
- GETUG-AFU 29 PEACE 3
- GETUG-AFU 30 ART STUDY
- GETUG-AFU 33 CARLHA 2
- GETUG-AFU 35 BLADDER SPARING
- GETUG-AFU 36 PEACE PRESTO
- GETUG-AFU 37 ALBAN
- GETUG-AFU 39 RAMPART
Strategic priorities
The current research strategy of GETUG focuses on three major axes :
- Prioritisation of trials that are highly competitive and have the potential to be “practice changing”
- Valorisation of past clinical trial
- New treatment strategies:
- Non irradiating local treatment
- Integration of imaging in research projects
- Promote the “DNA repair” area
Governance
GETUG acts under the governance of a steering committee, which is composed of members from various specialties and with relevant experience in urogenital cancer clinical trials. Members represent the various institutions (FCCC, University Hospital, private hospitals) participating in clinical trials and in the definition of the group’s strategy.
The main missions of this Steering Committee are to :
- Define the strategy of the Intergroup
- Propose and build prospective clinical trials & surveys or retrospective studies
- Validate the scientific value of the studies
- Promote the participation of all in cooperative work
- Collaborate with international cooperative groups
- Conduct national and international clinical studies in oncology, answering scientific questions on urogenital cancer.
- Identify and involve beforehand all experts in the design of the studies
Key projects
Peace 1
Peace 1 trial is a phase 3 trial with a 2×2 factorial design of abiraterone acetate plus prednisone and/or local radiotherapy in men with de novo metastatic castration-sensitive prostate cancer. This study is conducted on a European scale in around 100 sites with 1173 men were enrolled from Nov 2013 to Dec 2018. Sources of funding are the following: Janssen Pharmaceutical NV, Ipsen, Sanofi, PHRC. The first result of the primary analysis on rPFS will be presented to ASCO congress 2021 (oral session).
Alban
The Alban trial, which has already included more than 220 in France is funded by Roche. It is designed as an open label, randomized, phase III trial, evaluating efficacy of Atezolizumab in addition to one year BCG (Bacillus Calmette-Guerin) bladder instillation in BCG-naive patients with high-risk non-muscle invasive bladder cancer. The trial will be set up in Belgium, Germany and Spain with an objective of 516 patients included in Europe.
Peace 6 UNFIT
GETUG is the international coordinating structure of the European phase 3 PEACE-6 UNFIT trial favouring crossborder cooperation. This trial is designed as a multicentre, randomised, double-blinded, placebo controlled trial, evaluating the efficacy and safety of ADT +/- darolutamide in frail men with castration-naïve de novo metastatic prostate cancer. This clinical trial is funded by Bayer and will be set up in 14 countries over the next 2 years.
How to propose a research project ?
The criteria for acceptance/refusal of a study/trial to be developped within UCBG are transparent and are as follows:
- Adequacy or not to the strategy of the Intergroup
- Innovation and originality
- Medical, technical and financial feasibility
The proposal must be written in the form of a synopsis, by e-mail to the " target="_blank" rel="noreferrer noopener">Unicancer Clinical Programme Lead
The synopsis should include: title and acronym, objectives, end points, inclusion and exclusion criteria, study design, treatments under study if applicable, scientific background, statistical hypotheses, estimated costs (if available) and type of financial support envisaged.
The synopsis must be written in English.
Proposals remain confidential within GETUG Steering Committee. All members have signed a confidentiality agreement.
The project leader will be invited to discuss his/her proposal during a steering committee meeting or during “Assemblée Générale”.
After validation, the trial can be implemented with the help of the UNICANCER R&D project unit.
Partners
International partners:
GETUG collaborates with has a very large network of university hospitals, general hospitals, CLCCs and private institutions in France and abroad and develops research projects of international dimension by establishing links with institutions in Europe and the United States (SOGUG, ICORG, EORTC, SAKK, MD Anderson, RTOG, UCL …etc).