- RWD
ADN circulant
Circulating DNA research programme
Unicancer, a pioneer in personalised medicine, is developing a research programme on circulating tumour DNA.
Thanks to new technologies, knowledge about tumour biology is having an increasing impact on the clinical management of patients. The impact is not only important at the time of cancer diagnosis, but also at the time of treatment selection and throughout the course of care.
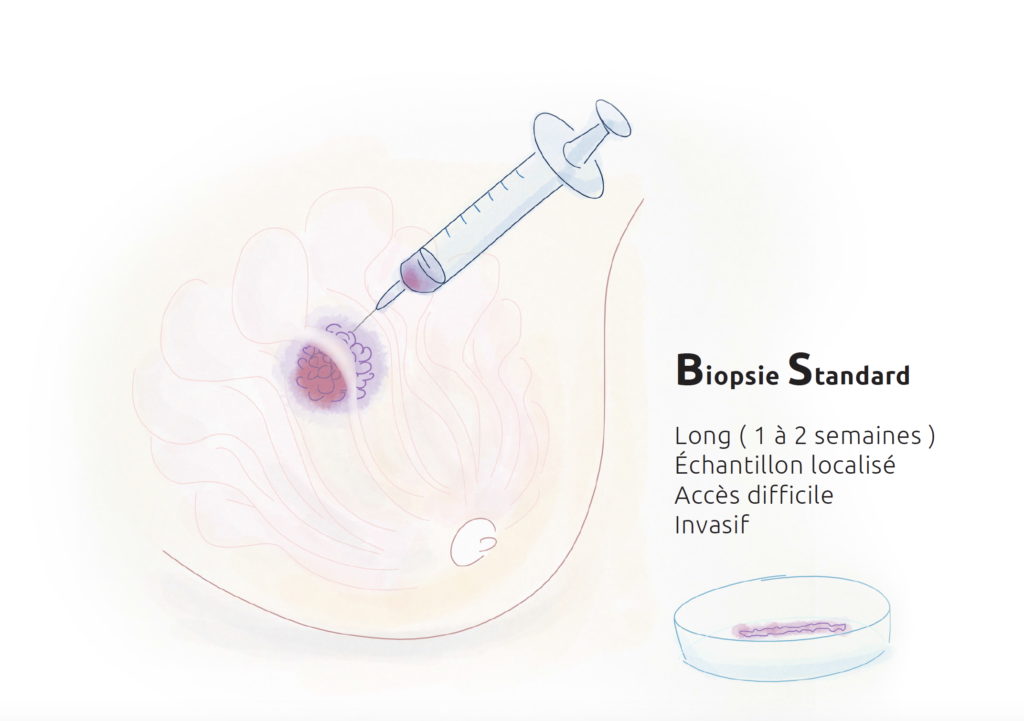
The increasing number of analyses to be performed on tumour samples for diagnostic and treatment decision purposes was becoming impossible to perform on tissue taken directly from the tumour (biopsy). This has prompted researchers to develop new strategies to guide management.
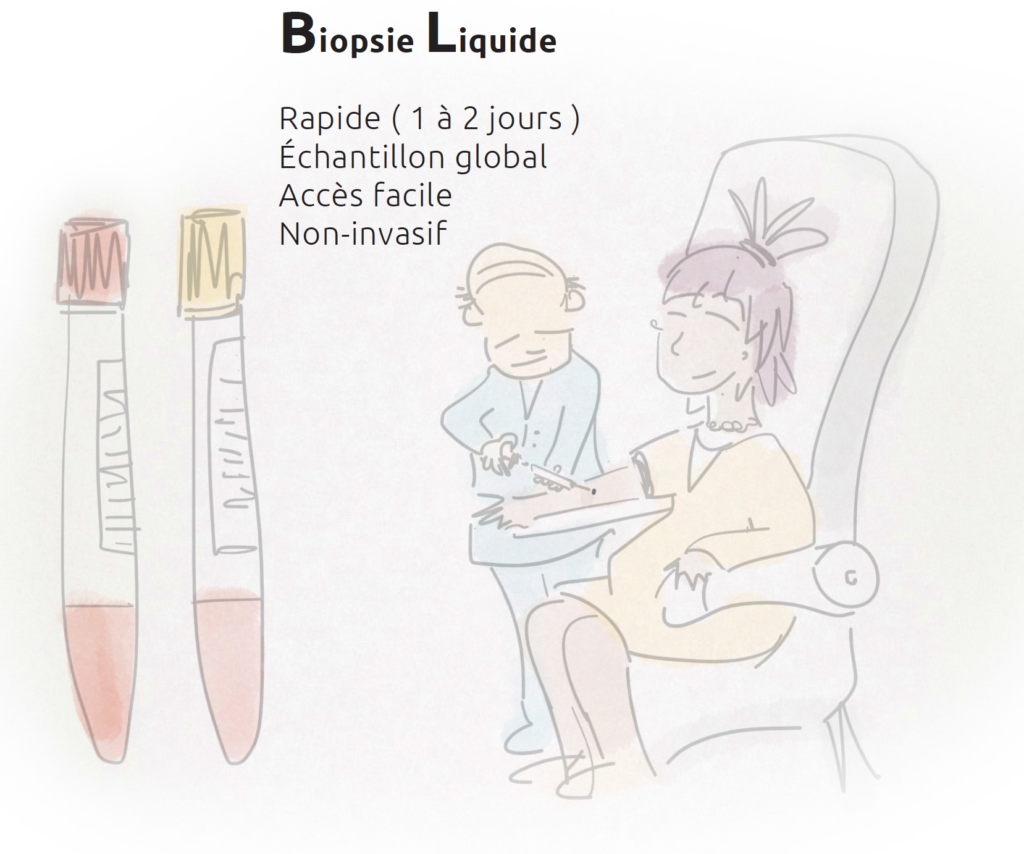
Key information can now be obtained from a blood sample (liquid biopsies), containing small amounts of tumour material circulating in the blood.
These new techniques represent an important step towards personalised oncology.
Circulating tumour cells (CTCs) and circulating tumour DNA (ctDNA) are two cancer-derived blood biomarkers that provide information on patient prognosis and treatment effectiveness in breast cancer.
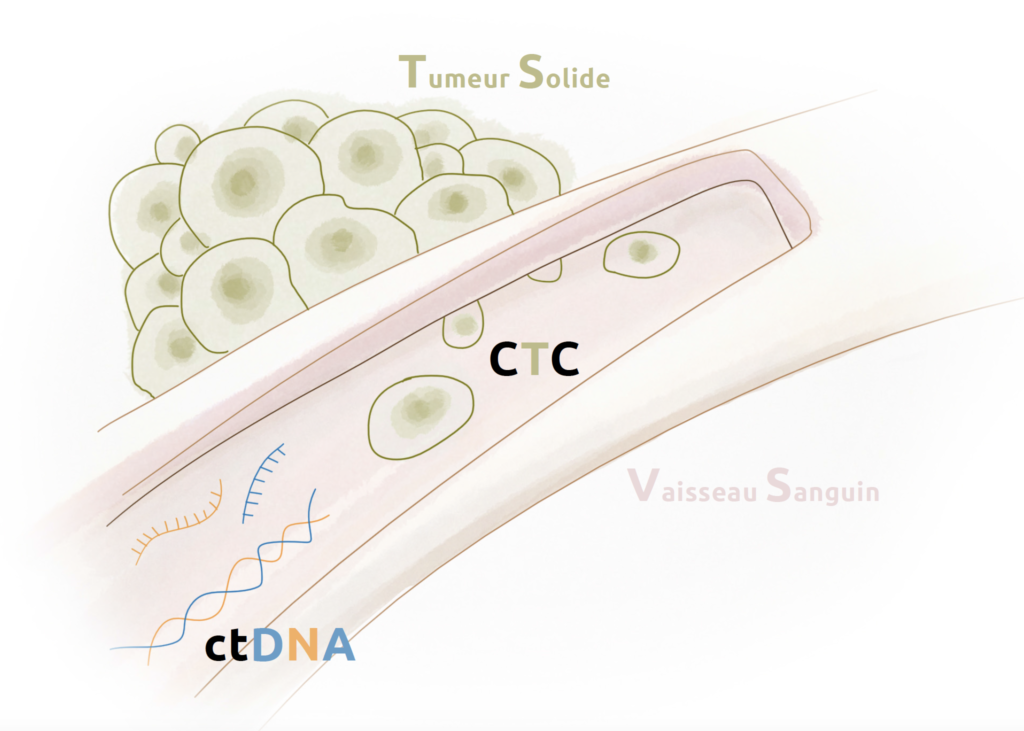
Several clinical studies or translational research projects are being conducted at Unicancer, in different indications, to better characterise and validate the clinical utility of these biomarkers.
COMET (NCT01745757)
Conducted in patients with metastatic breast cancer, this study indicates that CTCs and ctDNA have different detection profiles and complementary prognostic values (Silveira et al., 2021). Quantification of CTCs and ctDNA could help to establish the prognosis of patients and help to monitor the effectiveness of treatment.
Two large clinical trials are currently being conducted at Unicancer to determine whether changing treatment at the time of detection of biomarkers in circulating tumour DNA delays or prevents cancer progression or recurrence in patients at high risk of recurrence.
PADA-1 (NCT03079011)
Conducted in collaboration with the Arcagy-Gineco Intergroup, this study aims to evaluate the safety and efficacy of palbociclib, a drug directed against a molecule involved in cancer cell multiplication, in combination with a modification of hormone therapy in patients with estrogen-positive and HER-2 negative metastatic breast cancer.
Two types of hormone therapy will therefore be used in this study: an aromatase inhibitor that will be replaced by another class of drug called antiestrogen during the course of the study. The decision to change the hormone therapy from the aromatase inhibitor to the antiestrogen will be made on the basis of monitoring the increase in the frequency of occurrence of a mutation in the circulating tumour DNA (ctDNA) ESR1 gene in the blood of the participants (by means of simple blood tests).
Mutations in the ESR1 gene represent a mechanism of cancer cell resistance to aromatase inhibitor treatment and therefore precede the onset of disease progression in hormone receptor-positive metastatic breast cancer, which is the cancer under study.
The aim of the PADA-1 study is therefore to follow the evolution of mutations in the ESR1 gene in ctDNA in order to decide when to change the participants’ treatment to prevent their cancer from progressing or coming back.
TRAK-ER (NCT04985266)
This study, conducted in collaboration with the Royal Marsden Hospital in London, is a trial of early detection of metastasis by monitoring circulating tumour DNA (ctDNA) in participants receiving standard treatment for their ER-positive HER2-negative early breast cancer. After detection of ctDNA, a group of participants will be randomly assigned to receive either standard endocrine therapy (which may be modified from the therapy received before ctDNA detection) or palbociclib plus fulvestrant.
The trial is conducted in 2 phases:
- a surveillance phase during which participants are monitored for ctDNA,
- a treatment phase
Palbociclib and fulvestrant are two drugs already in use for the treatment of patients at high risk of developing breast cancer metastases:
- Palbociclib is a type of drug known as a CDK4/6 inhibitor. It has an impact on the growth processes of cancer cells and can stop them from multiplying.
- Fulvestrant is a hormone therapy that prevents oestrogen from causing breast cancer cells to grow and multiply.
The results of the ctDNA blood tests will tell participants whether they are at high or low risk of breast cancer recurrence. The aim of the study is to determine whether the use of ctDNA blood tests for monitoring cancer recurrence will lead to earlier detection of a cancer recurrence and whether the change in treatment triggered by ctDNA detection will lead to better outcomes for participants.
The results of this trial could change the way oncologists monitor and treat patients with ER-positive HER2-negative early breast cancer and improve their chances of not having their cancer return.